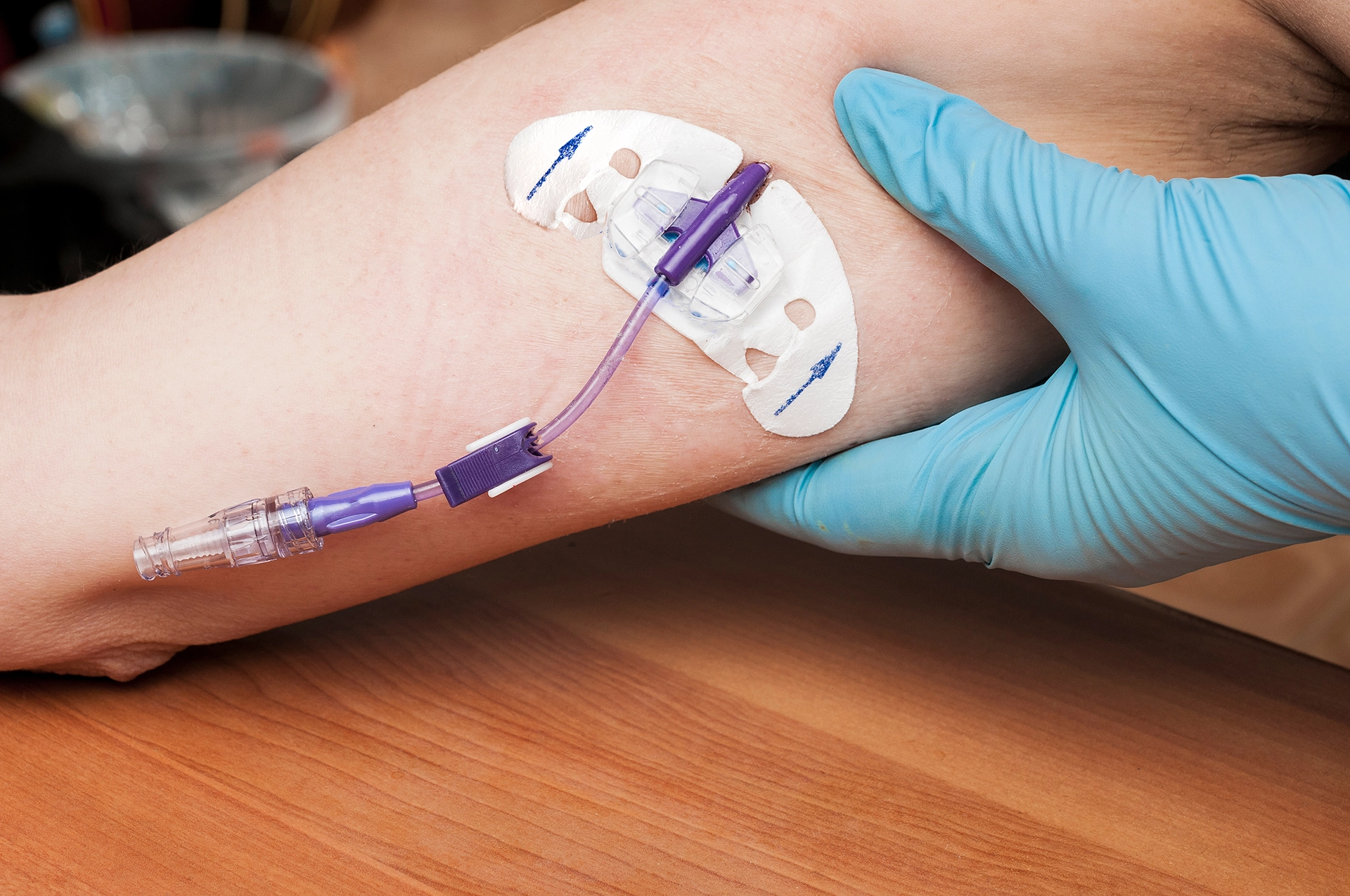
Every year, over 5 million central lines are inserted in the U.S.—a growing number being PICC lines and midlines. These devices are defectively designed. They expose patients to an unreasonably high risk of blood clots and infections, even though safer, coated alternatives have been available since 2013.
A 2020 study involving 3,560 patients found alarming outcomes:
- 28% developed sepsis
- 7% went into septic shock
Another 2016 study showed that 37.5% of patients had a catheter-associated blood clot within 5–14 days—far higher than manufacturer warnings suggest. Blood thinners did little to prevent these clots.
What Are PICC Lines and Midlines?
- PICC Line: A peripherally inserted central catheter placed through the veins of the arm, threaded toward the heart.
- Midline Catheter: A peripheral catheter inserted in the upper arm with the tip resting in the brachial or axillary vein.
Common Uses:
- IV antibiotics
- Total parenteral nutrition (TPN)
- Chemotherapy
- IV hydration
Complications We Litigate
We represent patients of all ages—from neonates to seniors—who’ve suffered from:
- Thrombosis or blood clots caused by PICC or midline catheters
- Bloodstream infections linked to catheter-associated biofilms and clots
- Catheter occlusion, requiring clot-busting drugs or device removal
- Device failure, such as kinking or cracking
- Catheter migration requiring surgical or endovascular removal
This is a product liability lawsuit. Manufacturers have ignored the availability of advanced coatings that reduce clotting and infections. Instead, they’ve continued to market outdated, unsafe devices—putting profits over patient safety.
The Real Cost of PICC Line and Midline Injuries
Catheter-related complications cost the U.S. healthcare system $4.5 billion annually.
- Each thrombosis complication: $24,558
- Each bloodstream infection: $12,982
- Each catheter occlusion: $624
These injuries aren’t minor:
- Blood clots can lead to pulmonary embolism, stroke, or death
- Infections can escalate into sepsis or septic shock, causing brain injury, kidney failure, or amputations
- Neonatal sepsis from uncoated catheters increases the risk of cerebral palsy in preemies
Manufacturers Must Be Held Accountable
Since 2012, manufacturers have known their polyurethane and silicone catheters degrade, migrate, clog, crack, and cause infections. They also knew about safer coating technologies—and still refused to use them. That choice demands accountability.
Manufacturers include:
- CR Bard Inc. (PowerPICC, PowerGroshong, etc.)
- Angiodynamics/Navilyst (Vaxcel, Xcela)
- Teleflex (ArrowEvolution)
- Medcomp (Vascu-PICC II, Pro-PICC CT)
- Cook Medical (Spectrum Turbo-Ject)
These companies failed to reduce inflammation, clot formation, and degradation risks—despite available solutions.
Compensation May Include:
- Medical costs for treating clots and infections (past and future)
- Pain and suffering
- Lost wages
- Punitive damages for knowingly selling unsafe devices
Neonatal Risks
Uncoated PICC lines in premature infants can lead to neonatal sepsis, which is a major contributor to cerebral palsy. Infections during this vulnerable stage can severely impact brain development. Prompt treatment and safer catheter design could prevent these devastating outcomes.
You Have Rights. We’re Here to Fight for Them.
We want to hear your story.